Ernest Rutherford
Ernest Rutherford was born on August 30, 1871 in Brightwater, New Zealand. He had passed away on October 19, 1937 . His wife was named Mary Georgina Newton. Ernest Rutherford attended Havelock School. Soon after he had gone to Nelson College where he proceeded to win a scholarship to attend Canterbury college, University of New Zealand. Ernest Rutherford went on to become a well respected chemist and has won many awards such as the Nobel Prize in Chemistry, Copley Medal, Elliott Cresson Medal, Franklin Medal, Rumford Medal, Matteucci Medal, Faraday Medal, and the Faraday Lectureship Medal.
He was awarded for the Nobel Prize in Chemistry in 1908 and moved in 1907 to the University of Manchester, where he proved that alpha radiation is helium nuclei.
He was widely credited with first splitting the atom in 1917 in a nuclear reaction between nitrogen and alpha particles in which he also discovered and named the proton. Rutherford became Director of the Cavendish Laboratory at the University of Cambridge in 1919.
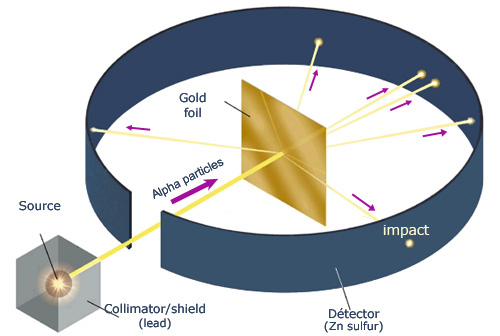 |
| A diagram of the Gold Foil Experiment |
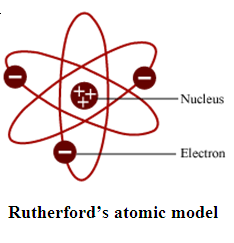 Rutherford’s most successful experiment was the Gold Foil Experiment, he shot alpha rays through gold foil and hypothesized that the alpha rays would be completely deflected because the paradigm at the time was the "plum pudding" model in which the atom was thought to be composed of electrons. When he did the experiment most rays passed through the foil, baffling him, while only some were repelled and knocked back, proving that like forces repel and opposite forces attract. He concluded that 99% of the mass of the atom is located in the nucleus, that the nucleus is minuscule, and that the charge of the nucleus is positive. In summation he disproved the "plum pudding" model and proved the existence of the nucleus in the atom.
Rutherford’s most successful experiment was the Gold Foil Experiment, he shot alpha rays through gold foil and hypothesized that the alpha rays would be completely deflected because the paradigm at the time was the "plum pudding" model in which the atom was thought to be composed of electrons. When he did the experiment most rays passed through the foil, baffling him, while only some were repelled and knocked back, proving that like forces repel and opposite forces attract. He concluded that 99% of the mass of the atom is located in the nucleus, that the nucleus is minuscule, and that the charge of the nucleus is positive. In summation he disproved the "plum pudding" model and proved the existence of the nucleus in the atom.
http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1908/rutherford-bio.html
http://www.chemheritage.org/discover/online-resources/chemistry-in-history/themes/atomic-and-nuclear-structure/rutherford.aspx
